Sodium sulfite (also known as sodium suplhite in the British English spelling) is a common food additive and preservative which has been widely used in the food industry for decades.
However, recent studies have raised concerns about its potential health risks. From allergic reactions to respiratory issues, it’s important to be aware of the potential dangers of consuming it.
In this article, we’ll dive into the top 5 health risks associated with this food additive and what you can do to protect yourself.
Table of Contents
Sodium Sulfite: A Brief History
Let’s take a quick look at who was involved in the process of discovering and isolating this compound.
Circa 1625 Johann Glauber, a German-Dutch alchemist, and chemist discovered sodium sulfite. According to one account, it is believed that he did this by boiling saltpeter (potassium nitrate) with sulfuric acid and then neutralising the resulting solution with soda (sodium carbonate). 1
However, it is argued that it is more likely that the process involved reacting sulfur dioxide, sodium carbonate, and water with the resulting substance (sodium bisulfite) being treated with sodium carbonate. 2
As much as Glauber was able to achieve this, many would agree that attributing the discovery of this to him is somewhat erroneous as he didn’t isolate it in a pure form.
In 1790 Nicolas Leblanc discovered a method for manufacturing soda ash (Sodium Carbonate) from common salt (Sodium Chloride). This became known as the Leblanc Process. Sodium sulfite is a byproduct of this process. 3 4
In 1826 Antoine Bussy was credited for the first isolation of it in its pure form. 5
It is important to note that, while Nicolas Leblanc’s process produces sodium sulfite as a byproduct, it was not his main goal and he did not isolate it in its pure form. The same can be said for Johann Glauber who is credited with the discovery of it.
Bussy’s discovery was particularly significant in that it marked the first time that a pure form had been produced and isolated, thus allowing for more accurate analysis and characterisation of the compound.
His work laid the foundation for further research into the properties and uses of this innovation, including its use as a food additive and preservative.
It isn’t clear at this stage whether the substance was isolated and used by anyone else as a food additive before Bussy’s discovery in 1826. However, it is likely that the preserving properties of sulfur dioxide, which is the main component of sodium sulfite, were known for centuries before Bussy’s discovery.
Sulfur dioxide is a common food preservative that has antimicrobial properties, which help to prevent spoilage caused by bacteria and fungi.
The use of sulfur dioxide as a food preservative dates back to ancient times, and it has been used in the preservation of a wide variety of foods, including dried fruits, wine, and beer. 6
Sulfur dioxide is also a strong antioxidant that helps to prevent the oxidation of fats, which can lead to rancidity. The use of sulfur dioxide in food preservation has been regulated by the FDA since the early 20th century.
The FDA has set limits on the amount of sulfur dioxide that can be used in various food products, and it is generally recognised as safe (GRAS) when used by good manufacturing practices. 7
The use of sulfur dioxide as a preservative is mentioned in ancient texts from Rome, Greece, and China. It was used as a fungicide and as a way to preserve wine, dried fruits, and meat.
It is possible that other forms of sodium sulfite were used as food preservatives before Bussy’s discovery, but the lack of a pure and easily-analysable form of the compound would have made it difficult to study or regulate its use.
Since then, it has had a long history of use as a food additive and preservative. It was first used in the late 19th century as a means of preserving dried fruits, and quickly became a popular ingredient in a wide range of food products.
Why use Sodium Sulfite as a Preservative instead of Sulfur Dioxide?
The argument for sodium sulfite over sulfur dioxide goes a little like this. Although sodium sulfite and sulfur dioxide are both used as preservatives in food, they do have some differences in their properties and benefits.
A few of the production-related reasons for using sodium sulfite over sulfur dioxide as a preservative for foods are:
- Sodium sulfite is more stable
- It is more stable than sulfur dioxide and is less likely to degrade or lose its effectiveness as a preservative over time. This means that it can be used in food products with a longer shelf life.
- Sodium sulfite has a broader range of uses
- It is considered to be a more versatile food preservative that is capable of being used in a wider variety of food products.
- Sodium sulfite has a lower allergenic potential
- It is less likely to cause allergic reactions than sulfur dioxide, which can cause allergic reactions in some people.
It’s worth mentioning that this compound is not as strong as sulfur dioxide in terms of antioxidant properties, and it can’t prevent the oxidation of fats.
Did you know that sodium sulfite was also used as a bleaching agent for textiles and paper? 8
Where does Sodium Sulfite come from?
This substance is a chemical compound that can be produced through the reaction of sulfur dioxide gas with a sodium compound, such as sodium carbonate or sodium hydroxide.
It is also produced as a result of the pulping process used to make paper.
In the Kraft process, which is the most common process used to make paper, wood chips are cooked with a solution of sodium hydroxide (NaOH) and sodium sulfide (Na2S) to break down the hemicellulose and lignin found in the wood.
This process produces a black liquor that contains dissolved organics and inorganic chemicals, including sodium sulfite. The black liquor is then processed to recover the chemicals and energy, and the sulfite is often used as a byproduct in the papermaking process as a bleaching agent and preservative. 9 10

Did you know that it can be found naturally in some mineral springs and volcanic gases? Mineral springs containing naturally occurring sodium sulfite can be found in various places around the world.
In the United States, mineral springs with naturally occurring sodium sulfite can be found in California, Colorado, Arizona, New Mexico, Utah, Nevada, and Wyoming. Other countries with mineral springs that contain naturally occurring sodium sulfite include Peru, Mexico, Germany, and France.
However, in most cases, the concentrations and accessibility of this naturally occurring substance are such that they are too low and difficult to secure to be commercially viable. Hence, the manufacturing of the substance through chemical processes.
How do you Make Sodium Sulfite?
It can be made through the chemical reaction of sulfur dioxide and sodium hydroxide. The process is typically done in a reactor and the reaction is exothermic, which means it releases heat.
The basic reaction is: SO2 + 2NaOH → Na2SO3 + H2O
Sulfur dioxide gas (SO2) is reacted with an aqueous solution of sodium hydroxide (NaOH) to produce a solution of sodium sulfite (Na2SO3) and water (H2O). The reaction can be catalysed by using a small amount of sulfuric acid or by adding a small amount of sodium bisulfite to the reaction mixture. 11 12
Or, the generalisation of the chemical reaction for sodium sulfite (without H20) is: SO2 + Na2CO3 → CO2 + Na2SO3.
Where the reaction between sulfur dioxide and sodium carbonate to produce sodium sulfite and carbon dioxide can be carried out in several ways, depending on the specific conditions and equipment used.
One common method is to mix a solution of sulfur dioxide and a solution of sodium carbonate, and then heat the mixture. The heat causes the reaction to occur, and the products – sodium sulfite and carbon dioxide – are formed.
The reaction can also be catalysed by the presence of certain compounds, such as sulfuric acid. The reaction can also be carried out in a reactor with a specific type of catalyst. The byproduct CO2 can be separated by bubbling through water. 13
It can also be made by dissolving sodium carbonate with sulfur dioxide, this is called the Solvay process.
It’s important to note that the production of this compound requires the handling of chemicals and it should be done under professional and safe conditions. It’s not recommended to make this at home.
What is the Difference Between Sodium Sulfite and Sodium Sulfate?
Sometimes these two get mixed up, and it’s easy to see why for those of us who are chemically uninitiated when their spelling is so similar. However, just one letter difference can mean dramatic differences in chemical structure and end-use cases.
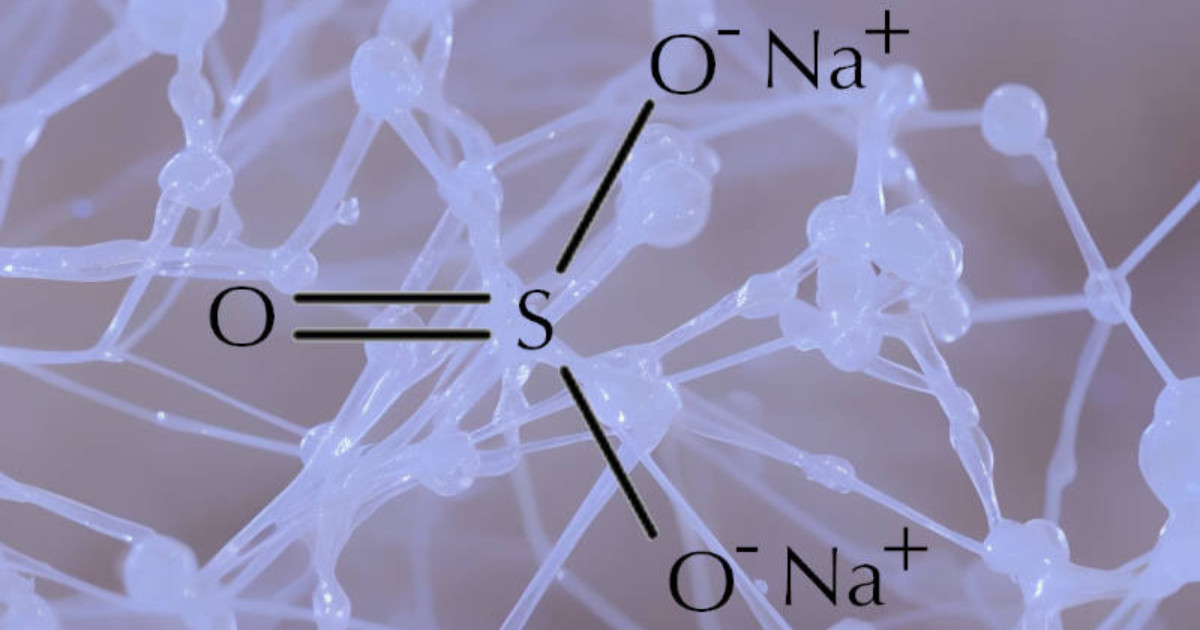
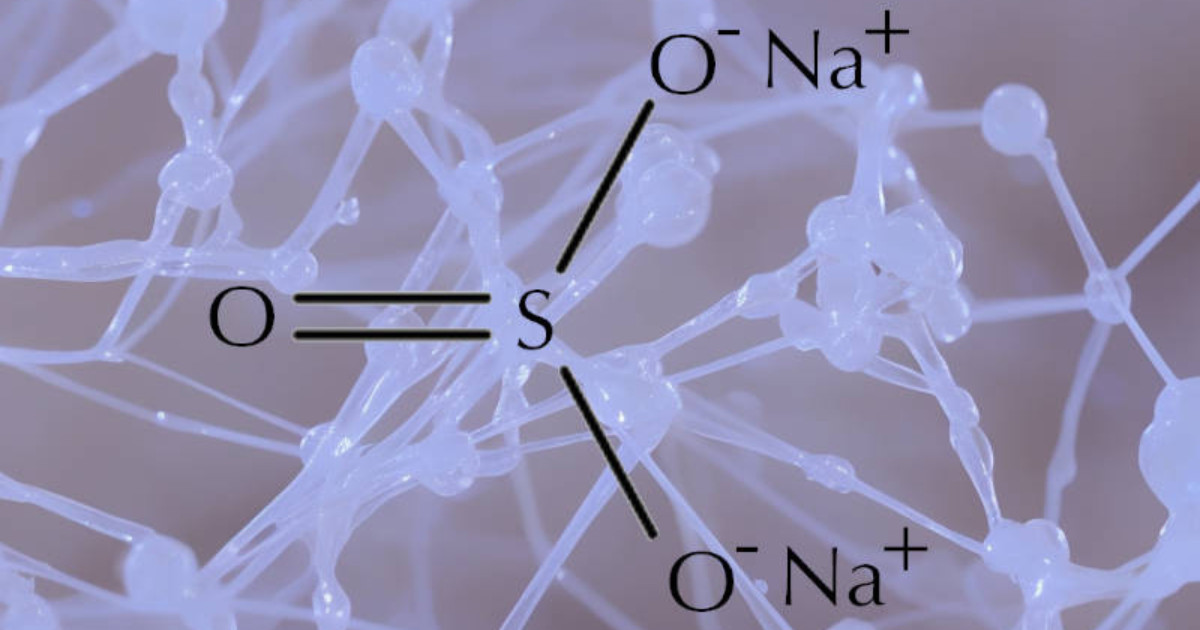
Sodium sulfite and sodium sulfate are two different compounds that have some similarities but are also distinct in their properties and uses.
Sodium sulfite (Na2SO3) is a white, crystalline powder that is a preservative and antioxidant that is commonly used in the food industry.
It is also used in the production of paper, textiles, and other products. The chemical is white and is soluble in water.
Sodium sulfate (Na2SO4) is a white, crystalline powder that was used as a laxative soon after its discovery, and in various manufacturing and industrial processes. 14
It is also used as a filler in some products, such as laundry detergents. It is a white crystalline solid and is also soluble in water.
While both are compounds containing sodium, sulfite and sulfate have different properties and uses. Sulfite is used as a preservative and antioxidant, while Sulfate is used in the production of glass, detergents, and textiles, and as a filler in some products.
Although they may sound similar, they have very different use cases and applications.
How Sodium Sulfite is Used Today
It is used in a variety of applications and industries today, including:
- Papermaking
- It is used as a bleaching agent in the papermaking process to remove colour from the paper.
- Food Preservation
- It is used as a preservative in foods such as dried fruits, canned vegetables, and wine to prevent spoilage and preserve colour.
- Photography
- It is used in the developing process of black and white films to fix the silver halides. Yes, people still develop film and photos!
- Water Treatment
- It is used as a reducing agent in the treatment of water to remove dissolved oxygen, which can be harmful to aquatic life.
- Pharmaceuticals
- It is used in the production of some medications, such as antibiotics and antihistamines.
- Other Industries
- It is also used in the textile, leather, and rubber industries as a bleaching and dechlorinating agent, and in the production of other chemicals such as thiosulfates and dithionites.
It’s worth noting that while this substance has many uses, it’s not a widely used chemical and its applications may vary depending on the region and industry.
In some countries, the use of sodium sulfite as a food additive is restricted or prohibited.
You might also want to take a look at some other considerations with water in our article that looks more closely at the topic of ‘Why do they put fluoride in the water?’.
5 Sodium Sulfite Health Risks
Can sulfites make you sick? They can cause allergic reactions in some people, such as hives, difficulty breathing, and stomach upset.
However, sulfite sensitivity is relatively rare, and most people can consume sulfites without any problems. It is estimated that less than 1% of the population is sensitive to sulfites. Just because the percentage number seems low, it doesn’t mean that the effects of sulfites are negligible for those who are impacted by them. 15 16
Is sodium sulfite bad for you? That depends on the amount you are exposed to, how long for, and in what form. Based on some of the studies listed below, the most problematic form of exposure is when it is in a micro-particle airborne form.
Given that this is not how most consumers would be exposed to this chemical, the determinations made on the safety may not be as poorly founded as some would argue.
However, that is not to say that I’m going to jump right into having products loaded with this type of chemical it in my home. Also, I would applaud efforts to further study the safety of this going forward.
Even though it is generally regarded as safe when used in food, it can cause adverse reactions in some individuals. These reactions can include:
- Nausea
- Vomiting
- Abdominal pain
- Dizziness
- Fatigue
- Headache, and
- Skin irritation.
In rare cases, it may cause anaphylactic shock and asthma attacks in some people.
Long-term exposure to high levels of this chemical is also suspected to increase the risk of certain types of cancer. Note that the language here is to “increase the risks”, not “cause”. We will look at that in a later section.
Asthma Attacks
This sulfite can trigger asthma attacks in sensitive individuals by causing bronchoconstriction, which is the narrowing of the airways in the lungs. This can make it difficult to breathe and can cause symptoms such as wheezing, coughing, and shortness of breath. 17 18
When an individual with asthma is exposed to it, the sulfite can bind to receptors in the airways, causing the muscles in the airway walls to contract. This leads to inflammation and narrowing of the airways, making it harder to breathe.
Additionally, sulfites can also cause the release of histamine and other inflammatory chemicals, which can further worsen bronchoconstriction. 19
It is important to note that not everyone with asthma will have a reaction to sulfites, and only a small percentage of people with asthma are sensitive to them. It pays to still be careful as this is not an enjoyable experience to encounter.
If you have asthma and suspect that sulfites may be triggering your asthma attacks, it is important to speak with your doctor. They may be able to help you identify the triggers and develop a plan to manage your asthma.
Anaphylactic Shock
Anaphylactic shock can be triggered by exposure to certain allergens, including sulfites. This sulfite can cause anaphylactic shock in sensitive individuals by activating the body’s immune system.
When an individual with a sulfite allergy is exposed to it, their immune system recognises it as a foreign invader and releases histamine and other inflammatory chemicals. These chemicals cause a cascade of reactions throughout the body, including the narrowing of the airways, swelling, and increased blood flow to the affected areas. 20
In severe cases, anaphylactic shock can cause a drop in blood pressure, rapid heartbeat, difficulty breathing, and even loss of consciousness. It is a medical emergency, and prompt treatment with epinephrine is essential to prevent severe consequences.
It’s important to note that anaphylactic shock is a rare reaction to sulfites and most people can consume sulfites without any problems. If you have a history of allergic reactions or suspect that you may be allergic to sulfites, it is important to speak with your doctor, who can help you identify the triggers and develop a plan to manage your condition.
Pulmonary Edema
Over three days, rats were studied as they were exposed to a fine aerosol of the chemical. They were found to have developed pulmonary edema along with tracheal epithelium irritation. 21
As this was an aerosolised version of the sulfite, it is reasonable to say that the average person is unlikely to be exposed to the same outcomes where the form of exposure is in foods or personal care products that are not aersoloised.
Fetal Toxicity
Referencing the same study cited in the section above, a review of a range of chemicals, including sodium sulfite, found that there were cases of fetal toxicity in rats where their exposure was through a large dose of heptahydrate form.
After this review, the panel determined that there were no significant risks associated with the use of this chemical in cosmetics.
Even with the 1995 decision of the Cosmetics Directive of the European Union to allow a maximum of 0.2%, expressed as free SO2, it may be worth reconsidering your use of products containing this chemical until you have been satisfied that it is safe for you.
Carcinogens (Really?)
Some Researchers advise that there is currently no evidence to suggest that it is carcinogenic or that it causes cancer.
However, as with any chemical, long-term exposure to high levels of this sulfite could potentially be harmful and more research is needed to determine the effects of prolonged exposure to the chemical and any associated increase in risks.
It’s also worth noting that some studies have suggested that individuals who consume high levels of sulfites, either naturally or through food additives, may have an increased risk of developing certain types of cancer, such as colon cancer, however, these findings are not conclusive and more research is needed to support confirmation. 22
It’s always recommended that you should be aware of the amount of sodium sulfite that you are consuming and follow the recommended guidelines for its use.
The International Agency for Research on Cancer (IARC) advises there are no links, many feel that further research is needed to better understand the potential link between the compound and cancer. And several studies consistently point to this being substantiated. 23 24 25
In particular, a contrary proposal I would like to draw your attention to comes from a 2006 study that found that mice lacking the Nrf2 gene had an increased susceptibility to dextran sulfate sodium-induced colitis. 26
Food for thought. That’s all.
5 Tips to Make Informed Choices with Sodium Sulfite
When it comes to making choices with products containing this chemical, it pays to:
- Check food labels
- Always check the ingredient list for “sulfites” or “sodium sulfite” when purchasing packaged foods. Sulfites are commonly used as a preservative in dried fruits, wine, and processed foods.
- Pro tip: download the Yuka app and scan the barcode of the item you are considering buying for a quick summary of potential risks and identifying smarter and healthier choices.
- Ask questions when eating out
- If you’re unsure about whether a dish contains sulfites, ask the server or chef. Many restaurants use sulfites in their kitchen, so it’s always a good idea to ask.
- Opt for fresh foods
- Fresh fruits, vegetables, and meats are less likely to contain sulfites than processed foods.
- Look for alternative preservation methods
- Some food manufacturers use alternative preservation methods, such as ascorbic acid (vitamin C) or citric acid, instead of sulfites.
- Consult with your doctor
- If you suspect that you may be sensitive to sulfites, consult with your doctor. They may be able to recommend alternative foods or treatment options that can help you avoid sulfites.
It is important to note that it’s always better to be safe than sorry and if you suspect you may have an allergic reaction to sulfites, it’s important to consult with a medical professional. They can help you identify the triggers and develop a plan to manage your condition.
FAQs
What is Sodium Sulphite used for?
It is a chemical compound that is used primarily as a preservative in food and as a reducing agent in various industrial processes.
It is also used in the production of wine, beer, and other fermented products to prevent spoilage and oxidation. In industrial settings, it is used as a reducing agent in the production of textiles, paper, and other products.
It can also be used as a bleaching agent in the pulp and paper industry and as a component of cleaning solutions.
Is Sodium Sulfite Toxic?
This sulfite can be toxic if ingested in large amounts, or if a person is sensitive or allergic to it. It can cause symptoms such as skin rashes, hives, itching, difficulty breathing, and swelling of the face, lips, tongue, or throat.
Ingestion of large amounts can cause nausea, vomiting, diarrhea, and in severe cases, shock. Long-term exposure to high levels of it in the workplace may also cause respiratory problems such as asthma.
However, when used in the recommended amounts, it is considered safe as a food preservative and in the industrial process. The FDA has set a limit on the number of sulfites that can be used in food.
In 1986, the FDA established a limit of 10 parts per million (ppm) for total sulfites in foods that are “likely to be consumed in a substantial quantity” and it’s also considered safe to consume as long as the appropriate limit is not exceeded.
It’s also recommended to avoid consuming sulfite if you’re allergic or have asthma and check the food label before buying to ensure it doesn’t have any added sulfite.
Is Sodium Sulfite Good for You?
This compound is not considered beneficial for human health when consumed in food or as a supplement. It is primarily used as a preservative and reducing agent in industrial processes, not as a nutritional supplement.
Although it is generally considered safe when used in the recommended amounts, excessive intake of it can cause negative health effects, as mentioned earlier. It’s not recommended to consume high levels of sulfite in food or supplements as it can cause allergic reactions, respiratory problems, and other health issues.
It’s important to read the food label before buying to ensure it doesn’t have any added sulfite if you’re sensitive or allergic to it.
What does Sodium Sulfite do to Your Hair?
It is not commonly used in hair care products, and there is limited research on its effects on hair specifically.
However, it is used as a reducing agent in hair dye formulas, which helps to prepare the hair for colouring by breaking down the natural pigment. It can also be used as a preservative in hair care products, but this is less common.
Is sodium sulfite bad for hair? Excessive use of hair dye or hair care products containing it can cause dryness, brittleness, and damage to the hair. It can also cause allergic reactions and itching on the scalp.
It’s important to check the ingredients label of the hair care product before buying and use it as directed.
It’s always a good idea to use hair care products in moderation and to use a moisturising conditioner after shampooing to help keep the hair healthy and hydrated. It’s also recommended to avoid using hair dyes or hair care products that contain it if you have sensitive skin, a history of allergies, or if you have dyed your hair in the past and experienced negative side effects.
What is the Formula for Sodium Sulfite?
The Formula for Sodium Sulfite is Na2SO3.
Conclusion
Choosing to find out what is in your food and other consumable products can be a daunting task. Through our initial look at the various chemical preservatives used in many products, you will hopefully feel more like you have better control over your choices.
Like with all things, moderation is quite often the key. Abstinence may be your preference, and if that’s how you roll, that’s fine. The important thing is that you keep learning, which is what I strive to do and build on your experience as you go.
Not all preservatives have a completely dark side. Some are simply neutral, for the most part. Does that mean I’m going to load up on products containing sodium sulfate? Hell no! But I’m not going to live in fear of it either.
Join the conversation on Instagram and Pinterest. Share your thoughts.
References
- “Johann Glauber” – W. B. Ashworth, 10 March 2020 [Linda Hall Library] [Archive] ↩︎
- “Chemical Compounds – Sodium Sulfite” – pp 785, N. Schlager, J. Weisblatt, D. E. Newton, C. B Montney, 2006 [The Vespiary] [Archive] ↩︎
- “Nicolas Leblanc; French Chemist” – The Editors of Encyclopaedia Britannica, 20 July 1998 [Britannica] [Archive] ↩︎
- “School Science Lessons; Topic 12A – 12.16.8 Prepare sodium carbonate, LeBlanc process” – J. Eflick, 10 August 2022 [University of Queensland] [Archive] ↩︎
- “Revista CENIC Ciencias Químicas, Vol. 43, 2012: Antoine Alexandre Brutus Bussy” – J. Wisniak, 22 September 2011 [Redalyc] [Archive] ↩︎
- “A Closer Look at Sulphur Dioxide in Foods” – S. Wong, February 2021 [Hong Kong Center for Food Safety] [Archive] ↩︎
- “CFR – Code of Federal Regulations Title 21: Sec. 582.3862 Sulfur dioxide.” – FDA Staff, 29 November 2022 [FDA] [Archive] ↩︎
- “Sodium sulfite” – CAMEO Staff, 2 June 2022 [CAMEO] [Archive] ↩︎
- “Chemical Wood Pulping” – EPA Staff, Last accessed 22 January 2023 [EPA] [Archive] ↩︎
- “Sulfite Process” – The Editors of Encyclopaedia Britannica, 20 July 1998 [Britannica] [Archive] ↩︎
- “SO2 + NaOH = Na2SO3 + H2O – Chemical Equation Balancer” – ChemicalAid Staff, Last Checked 22 January 2023 [ChemicalAid] [Archive] ↩︎
- “Sodium Sulfite- Na2SO3” – BYJU’S Staff, Last Checked 22 January 2023 [BYJU’S] [Archive] ↩︎
- “SO2 + Na2CO3 = CO2 + Na2SO3 – Chemical Equation Balancer” – ChemicalAid Staff, Last Checked 22 January 2023 [ChemicalAid] [Archive] ↩︎
- “The Renaissance of Science: The Story of the Cell and Biology” pp 288 – A. Martini, May 2015 [Google Books] [Archive] ↩︎
- “Sulfites: Human health tier II assessment” – NINCAS Staff, 28 June 2013 [Australian Industrial Chemicals Introduction Scheme] [Archive] ↩︎
- “Sulfites: Separating Fact from Fiction” – P. Grotheer, M. Marshall, A. Simonne, 11 May 2022 [University of Florida] [Archive] ↩︎
- “Sulfites: A Sensitive Issue” – American Academy of Pediatricians Staff, 29 December 2011 [Healthy Children] [Archive] ↩︎
- “Sulfite Sensitivity Frequently Asked Questions” – ASCIA Staff, July 2021 [ASCIA] [Archive] ↩︎
- “Adverse reactions to the sulphite additives” – H. Vally, N. LA Misso, 2015 [PubMed] [Archive] ↩︎
- “Food Allergies” – J E. Perkin, 13 August 2018 [Encyclopedia.com] [Archive] ↩︎
- “Final Report on the Safety Assessment of Sodium Sulfite, Potassium Sulfite, Ammonium Sulfite, Sodium Bisulfite, Ammonium Bisulfite, Sodium Metabisulfite and Potassium Metabisulfite” – B. Nair. A. R. Elmore, 24 March 2003 [Sage Publications] [Archive] ↩︎
- “Food additives promote inflammation, colon cancer in mice” – C. Torgan, 22 November 2016 [NIH] [Archive]
↩︎ - “Lesions Associated with Mineral Deposition in the Lymph Node and Lung of the Dog” – M. J. Day, G. R. Pearson, V. M. Lucke, S. J. Lane, R. S. J. Sparks, 1996 [Sage Publications] [Archive] ↩︎
- “Dipotassium disulphite” – ECHA Staff, Last Checked 22 January 2023 [ECHA] [Archive] ↩︎
- “Strontium Sulfite: A New pH-Responsive Inorganic Nanocarrier to Deliver Therapeutic siRNAs to Cancer Cells” – M. E. Karim, J. Shetty, R. A. Islam, A. Kaiser, A. Bakhtiar, E. H. Chowdhury, 16 November 2018 [MDPI] [Archive] ↩︎
- “Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis” – T. O. Khor, M. T. Huang, K. H. Kwon, J. Y. Chan, B. S. Reddy, A. N. Kong, December 2006 [PubMed] [Archive] ↩︎
Last Updated on 5 months by D&C Editorial Team


