I find it interesting thinking back now to the question I was asked recently ‘what is DMSO used for in humans?’ which led my mind to when I was a child. I can still see the little amber glass bottle of dimethyl sulfoxide (DMSO) my Grandfather had in his fishing tackle box.
Any time he had a scrape, cut, or nicked himself while out on the riverbank, this was his ‘go-to’ without fail. After he had stopped any bleeding, I saw the little bottle come out.
As a kid, I didn’t really think that much of it. It was just something he did. And it seemed to work. As an adult, I have come to gain a better understanding of his wisdom.
Side note, I’m pretty sure this particular brand of wisdom was something he gained from his wife. My Grandmother was a Naturopath who had a successful practice in Melbourne from the 1940s until just before she passed away in the early 1980s.
You know that old saying about every great man, right? Well, she certainly was a great woman.
This clear liquid has been around me in a quite number of ways since those days. Most recently as an ingredient in a mouthwash that my wife made for us. It is an incredibly versatile product, and it’s amazing what you find out it is used for once you start digging.
Recently, there seems to have been a revival of sorts in it being used for more applications than what it is generally sold for. This, therefore, requires some attention and specific care. With that said, please take a moment to read the Notice below.
Table of Contents
What is DMSO?
Dimethyl sulfoxide is an organic compound with the formula C2H6OS (at times listed as (CH3)2SO). Both formulas represent the same compound and are commonly used interchangeably.
It is a clear colourless liquid with a molecular weight of 78.13g/mol and a boiling point of 189°C. It freezes at about 18.5°C, which is why in cooler climates it can appear crystalised. 1 2 3
One interesting fact about this product is that it has the ability to penetrate through living tissue, including the skin, due to its small molecular size and polar properties.
This makes it a potentially useful substance for a variety of topical medications and skin care products, as well as a potential carrier for delivering drugs through the skin and into the bloodstream. More research in this area is required.4
What to do if it Freezes
Ok. So you’ve got a bottle that looks like a frozen block, and nothing wants to come out. Due to the freezing point for dimethyl sulfoxide being in the 18.5°C range, it can turn solid on a cool day when other things in your home simply do not freeze.
Don’t panic. Nothing’s wrong here, this is a normal chemical reaction with pure DMSO. If it has been diluted with other liquids, like distilled water, then it’s not likely to freeze this way.
To get it to thaw out again it is not advisable to raise the temperature too quickly. Certainly, avoid putting it in a microwave! This is not a good idea!
Defrosting it is best done by placing it in a warm location, like a sunny windowsill, or in a room where the temperature is above 20°C. You may also choose to place your bottle in a warm bowl of water to raise the temperature and return it to a fluid state.
Keep the lid on, and just give it some time to return to a fluid state.
Dimethyl Sulfoxide Uses; A Word of Caution
It is appropriate at this stage that I share a word of caution with you. Some people may opt to attempt any manner of self-administrative processes, which, if you do not have the requisite proficiency, skills, and knowledge, can be quite harmful.
DMSO has the property of enhancing the permeability of other molecules, including some medications, increasing their ability to pass through cell membranes. This means that it can increase the relative effects of some drugs when they are dissolved in dimethyl sulfoxide and applied to the skin or injected into the body. 5 6 7
Think of ‘enhancing the permeability’ in this way; in the case of medication, dimethyl sulfoxide can act as a carrier, allowing drugs to be delivered through the skin and into the bloodstream at much higher levels than they would be able to otherwise achieve on their own.
The “Higher Concentrations” Argument
Some things, like vitamin and mineral supplements, are made in much higher doses or strengths than they occur in nature. Take for example vitamin C in oranges versus tablet form. There is something to be said about choosing whole foods over tablets.
At this stage, if this topic is something new to you, it may be worth considering this concentration explanation connected to vitamin C rich foods before progressing further.
For those who get the gist of what is being applied here, let’s continue. For those who have just come back after reading the article linked above, welcome back!
So, with the increased concentration of active agents and other components in mind in a whole host of man-made pharmaceuticals and health-related products, let’s get back to how this links in with DMSO and a word of caution.
Lowering the Friction
This concept may be best described as ‘friction’ presented by an absence of suitable ‘keys for locks’ which is at times encountered by some medications, and other health-related products, requiring manufacture in higher concentrations so they can work per the objectives in the human body. The introduced presence of DMSO can have a significant impact on these.
Let’s unpack that.
Think of that friction as being the body’s natural mode of operation where certain functional components are not present. Like with the vitamin C mentioned in the article linked above, where potassium, folate, and magnesium are required to support the body’s effective use of vitamin C found in oranges.
In the absence of these components, the concentration of the active agent (e.g. vitamin C) needs to be that much higher in manufactured products to get through these “locks” it does not have the “keys” to.
DMSO in such situations can act as a figurative master key, bypassing this biological friction as a result of its nature.
Therefore, as less restrictive pathways are opened for other medications through the use of a master key like this, their potency from an “effect perspective” is arguably amplified. 8 9
This increased penetration and delivery of concentrations of drugs into the body can potentially increase the amplification of the medication. This may be a good thing in some cases, and not so good in others.
It may prove to be harmful in that medications are not broadly manufactured or prescribed in the specific concentrations that they are with the uncommunicated intention of the end user taking them in conjunction with DMSO.
Such situations may therefore result in an effective “overdose” where the resistance that the medication may normally encounter as a result of ordinary bodily functions is reduced due to the properties of the DMSO.
It’s worth noting that at the time of writing this article, DMSO has not been approved by the FDA or TGA for human use, and further research is needed to fully understand the extent to which it can enhance the delivery of drugs and other compounds through the skin and via other means. 10 11 12 13 14
For some people, this detail is a little confusing as even though it has not been approved for human use, it has been used in many medical applications. We’ll take a closer look at those in this article, and some considerations to keep in mind.
The Power of Sulfur
Dimethyl sulfoxide is an organosulfur (sulfur-containing) compound, and its unique properties are due to the presence of sulfur.
Did you know that sulfur is an important element for many chemical reactions and processes?
It is used as a reagent in the production of sulfuric acid, sulfur dioxide, and many other compounds. In the medical field, sulfur is used to make medicines, antiseptics, and preservatives. It is also used in the production of cosmetics, food additives, and insecticides.
Sulfur is essential for the synthesis of proteins, enzymes, and vitamins, and it is also essential for the metabolism of carbohydrates, proteins, and fats.
One study looked at the use of different sulfur compounds for the mass production of desulfurising cells. The results showed that using dibenzothiophene (DBT) as the main sulfur source was not practical due to its high cost and negative effects on cell growth.
However, using dimethyl sulfoxide (DMSO) as the sulfur source allowed for better growth and higher desulfurising activity. The study recommended using DMSO as the appropriate sulfur source for mass production of the studied bacteria, with improvements in desulfurising activity made by adding DBT later. 15
Sulfur (also spelled as Sulphur in British English) is capable of forming powerful hydrogen bonds, which allows it to dissolve a wide variety of substances.
Did you know that these hydrogen bonds make DMSO an effective solvent for organic compounds, and it can also be used to dissolve inorganic substances such as metals and salts? 16
The DMSO Back Story
DMSO is understood to have been first synthesised by the Russian chemist Alexander Zaytsev in 1866. Zaytsev discovered Dimethyl sulfoxide when he was attempting to oxidise dimethyl sulfide, and he found that the oxidation reaction yielded dimethyl sulfoxide as a byproduct.
Since then, it has been used for a variety of industrial and medical purposes with the most notable accounts being documented by Stanley W. Jacob M.D.
Jacob was a professor of surgery at the Oregon Health & Science University, and he is best known for his work on the medicinal properties of dimethyl sulfoxide. He discovered its therapeutic effects in the 1960s, and he published several papers and books on the topic, drawing widespread attention to the substance.
His work on this demonstrated the liquid’s ability to reduce pain and swelling, and he also showed that it could be used as a carrier to transport other substances through the skin and into the body.
This led to the development of new pharmaceutical formulations that combined dimethyl sulfoxide with other active ingredients, and Jacob is credited with pioneering the use of it as a therapeutic agent.
In addition to his research, Jacob was a passionate advocate for the use of dimethyl sulfoxide, and he devoted much of his career to promoting its use and educating the public about its potential benefits.
He was a controversial figure in the medical community, and his claims about the therapeutic effects of it were often met with skepticism. However, his work paved the way for further research on dimethyl sulfoxide and its potential uses. He is widely recognised as a pioneer in the field of DMSO research.
Where does DMSO come from?
The primary method of manufacturing DMSO is through the oxidation of dimethyl sulfide (DMS) obtained from crude sulfate turpentine, which is a byproduct of the Kraft pulping process of paper production. The oxidation of DMS can be carried out using a combination of oxygen and a catalyst.
The oxidation reaction takes place in a reactor vessel where the DMS and oxygen are mixed, and the catalyst is added. The reaction temperature is carefully controlled to prevent thermal runaway and the formation of byproducts. 17 18
The reaction is exothermic, which means it releases heat, so cooling is also required. After the reaction is completed, the DMSO is separated from the reaction mixture by distillation. The DMSO obtained from the distillation process is purified further by removing any residual impurities, including water and other byproducts, through additional distillation or other purification techniques.
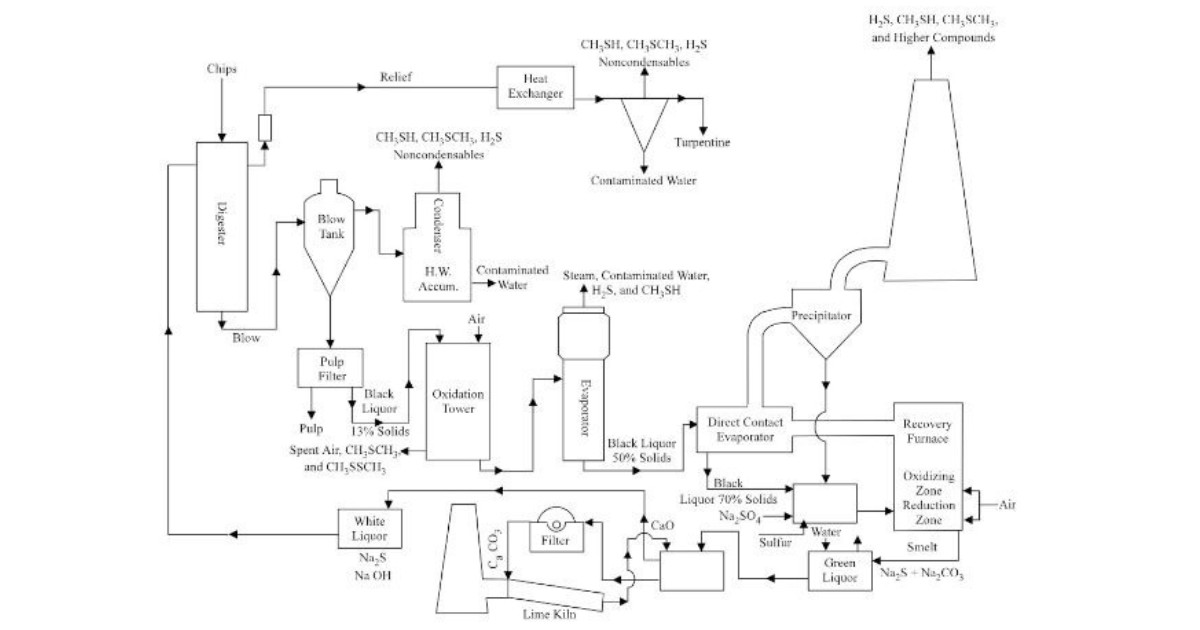
The purity of the final DMSO product depends on the specific use, but it is typically 99.9% pure or higher. The process of manufacturing DMSO is highly efficient, with yields of up to 99%.
The use of modern process control techniques and optimisation of reaction conditions has made it possible to produce large quantities of high-purity DMSO with a high level of consistency and reliability.
Where Lignin Fits In with DMSO
Lignin is a major component of wood and is closely related to the production of DMSO. Lignin is a byproduct of the pulping process used in paper production.
In the Kraft pulping process mentioned above, wood chips are treated with a mixture of sodium hydroxide and sodium sulfide to separate the cellulose fibres from the lignin.
The resulting lignin-rich black liquor is then burned to generate energy, and the waste gases, including dimethyl sulfide (DMS), are scrubbed and captured.
DMS is then further processed to produce DMSO. The DMSO manufacturing process involves the oxidation of DMS using a combination of oxygen and a catalyst.
The reaction is carried out in a reactor vessel, where the DMS and oxygen are mixed and the catalyst is added. The process is similar to that described above where controlling the reaction temperature aids in the prevention of thermal runaways and byproducts. 19
After the reaction is completed, the DMSO is separated from the reaction mixture by distillation, and the final product is purified to remove any residual impurities.
The Benefits and Uses of DMSO
As we explore the benefits of DMSO I would like to stress that this is not an encouragement to attempt any form of self-treatment without expert medical support or guidance. The previously mentioned Notice concerning this article stands.
This is simply a consideration of the proven studies, and research into the application of DMSO in controlled studies, and product manufacture.
Antiseptic Effects
One particular study has shown that it has been shown in concentrations of 4% to 5% increased the effectiveness of antiseptics. 20
Researchers evaluating the antiseptic effectiveness of dimethyl sulfoxide against bacteria commonly found in the urinary tract collected some interesting data.
An important note to keep in mind here is that the study was conducted in vitro, meaning it was done in a laboratory setting using test tubes and Petri dishes rather than in live subjects.
The Researchers tested the antiseptic activity of DMSO against a range of urinary tract bacteria, including:
- Escherichia coli
- Klebsiella pneumonia
- Pseudomonas aeruginosa, and
- Proteus mirabilis.
The results showed that DMSO had significant antiseptic activity against these bacteria, indicating that it may have potential as an antiseptic agent in the treatment of urinary tract infections.
Interstitial Cystitis Treatment
DMSO is well known for being used to treat interstitial cystitis/bladder pain syndrome (IC/BPS). This is a condition that causes pain in the bladder and pelvic region, along with frequent urination and discomfort.
It has been used to relieve symptoms like urgency and pain in IC/BPS because it has analgesic, anti-inflammatory, and muscle-relaxant properties.
In a study published in 2013, a 50% aqueous solution of DMSO, known as RIMSO-50 featured as an area of investigation. It is commonly used to treat the condition. 21
Studies have shown that it can penetrate tissues without causing harm, and can also be used to enhance the transport of chemotherapy drugs into bladder tumors.
In animal models, intravesical instillation of DMSO showed a direct correlation between the drug concentration and anti-inflammatory effects on the bladder. It is understood that it may work by influencing conduction and neurotransmission in sensory nerves, though more research is needed in this area.
Enhancing Medication Delivery Efficiency
The use of DMSO in transdermal medication, such as nicotine patches is providing for some interesting advances. One patent looks at it as being utilised as a penetration enhancer.
The patent highlights that it helps the active ingredient, nicotine, in the patch to be absorbed more efficiently through the skin and into the bloodstream.
This allows for a higher dose of nicotine to be delivered, and for the patch to be more effective in reducing withdrawal symptoms and cravings in smokers trying to quit.
The use of DMSO in nicotine patches has been shown to improve their efficacy in clinical studies, making it a valuable addition to the patch formula. 22
Another study conducted focused on preparing transdermal patches containing estradiol with varying concentrations of DMSO. The patches were then applied to the skin of rats, and the amount of estradiol delivered into the bloodstream was measured. 23
Evidence gathered through this study supported the use of dimethyl sulfoxide in transdermal patches for the delivery of estradiol. The study found that the addition of dimethyl sulfoxide to transdermal patches significantly increased the permeation of estradiol through the skin and into the bloodstream.
Based on the results of this study, one could argue that the use of DMSO in transdermal patches provides a useful strategy for improving the efficacy of transdermal patches for delivering estradiol and other drugs. The study found that the highest concentration of DMSO used (10%) was associated with the greatest increase in estradiol permeation.
Not Always an Effective Option
However, as good as it may be in some instances, it has proven not to be the case with certain platinum-based chemotherapy drugs. 24
Platinum-based chemotherapy drugs are commonly used to treat a range of cancers, but they can cause toxic side effects. In a study aimed at determining whether the addition of DMSO to these drugs would affect their effectiveness and toxicity Researchers found there were some issues.
The study tested the effect of DMSO on the activity of cisplatin and carboplatin against several cancer cell lines. The results showed that the addition of DMSO significantly decreased the activity of the platinum-based chemotherapy drugs and reduced their toxicity to normal cells.
The Researchers concluded that DMSO can inactivate platinum-based chemotherapy drugs, reducing their effectiveness and toxicity. Understandably, further research is needed to fully understand the impact of DMSO on platinum-based chemotherapy drugs and to determine the best ways to use these drugs in the treatment of cancer.
Cryobiology
DMSO has been widely used as a cryoprotectant in cryobiology for preserving living cells, tissues, and organs during freezing. It acts by reducing the formation of ice crystals, which are harmful to cells and can lead to cell death during the freezing process.
The efficacy of it as a cryoprotectant has been demonstrated in numerous studies, where it has been shown to increase the viability of cells and tissues after thawing. However, it does not come without its drawbacks. Various studies argue that the use of high concentrations of DMSO can lead to toxicity, causing damage to cells and tissues, and may even lead to cell death.
It is important to note that while DMSO is widely used in cryobiology, its efficacy and safety are still the subject of ongoing research. 25 26
The optimal concentration and method of administration of DMSO can vary depending on the type of cells, tissues, or organs being preserved, and must be carefully evaluated on a case-by-case basis.
Dimethyl sulfoxide has been a key player in cryopreservation technology for more than 40 years, serving as the cryoprotectant of choice for most animal cell preservation protocols. However, while its use has been established as safe and effective, its cytotoxicity raises concerns and is driving the search for alternative cryoprotectants.
The diversity and complexity of cell therapies demand new cryopreservation methodologies, including the ability to store and ship cell-based medicines in a viable yet stable state.
This is particularly challenging when dealing with non-frozen materials and when demonstrating product comparability across different manufacturing sites.
In this review, the Authors assess the use of DMSO in cryopreservation and evaluate the ways in which its toxic effects can be reduced, removed, or replaced. 27
They consider the real incidence of adverse effects in patients and the probable causes, as well as alternative cryoprotectants and their progression toward clinical use.
While DMSO remains the cryoprotectant of first choice, replacement options are being developed, though they have yet to make a significant impact in cell therapies.
Concerns with Human Cellular Processes
In the interests of a balanced and objective perspective, one particular study highlighted concerns about using DMSO as a solvent in biotechnological applications.
Their findings suggested that it can induce changes in cellular processes in both cardiac and liver cells, which can have negative consequences for conclusions drawn from cell assays and applications of these findings. 28
The extreme changes in miRNA and alterations in the epigenetic landscape caused by DMSO were described as also having the potential to pose a threat, particularly to assisted reproductive technology.
Global deregulation of methylation mechanisms induced by DMSO, especially when it affects genes important in development, is highlighted as possibly having negative consequences directly, later in life, or potentially in later generations.
Therefore, it was concluded that the use of DMSO should be avoided where possible, and if it is indispensable in biotechnological applications, its effects should be considered and the concentration should be kept as low as possible.
This is in direct contrast to the wording on the TGA website about genotoxicity or other aspects of toxicity which may be connected to reproductive or developmental health. 29
Stem Cell Transplantation – The Evolution
Although this is still cryobiology, I felt that it required an opportunity to be explored on its own. Stem cell transplantation and stem cell therapy are gaining more and more attention of late.
DMSO helps during the cryopreservation processes to protect mesenchymal stem cells (MSCs) from damage during freezing and thawing, which can be a critical step in the preservation of stem cell viability and effectiveness. The use of it in these processes is a current necessity, although alternative options are being explored.
One study into a DMSO alternative, known as betaine, provided some interesting results when they looked at working with MSCs. MSCs are used in medical treatments for various conditions and are stored in a frozen state to ensure long-term viability. 30
DMSO is used as a primary cryoprotectant in current protocols for MSC cryopreservation as it is easily permeable and protects the cells from cryo-injuries. However, it is regarded by many as being toxic in higher concentrations and can cause adverse effects on cell function.
To address these challenges, researchers have been investigating alternative biocompatible materials to replace DMSO, including trehalose, plant proteins, and biotechnologies such as microfluidic-based hydrogel microencapsulation, droplet-based cell printing, and nanowarming.
The study found that zwitterionic betaine is one alternative that has been found to have excellent compatibility and cryoprotective properties while reducing levels of harmful reactive oxygen species.
Further research is needed to assess the impact of these alternative methods on the in vivo characteristics of MSCs.
Anti-Inflammatory and Pain Relief Potential
Conditions such as arthritis can bring with them severe levels of pain for those suffering from it. A study published in 2016 looked at the repression of inflammatory cytokine production from blood cells using DMSO.
One of the hurdles that proved to be a challenge with a true double-blind study was the garlicky odour one can find in their mouth after using DMSO. The results of the study found that it demonstrated efficacy in whole human blood by inhibiting specific signaling pathways in human monocytes and reducing the production of inflammatory mediators. 31
Linking this to the reduction potential for arthritic events in mouse models found there was a benefit in using DMSO for its anti-inflammatory properties.

Wound Healing Potential
A study was conducted to examine the effects of low-concentration dimethyl sulfoxide on skin wound healing. The researchers created small incisions on the backs of mice and then applied low-concentration dimethyl sulfoxide to one of the wounds, while the other wound served as a control.
The wounds were monitored over a period of time, and it was found that the wound treated with low-concentration dimethyl sulfoxide healed significantly faster than the control wound. 32
The study showed that low-concentration DMSO not only accelerates the healing process but also improves the quality of the healed tissue.
The researchers observed increased production of collagen, a protein that is essential for the formation of new tissue and provides strength to the skin, in dimethyl sulfoxide-treated wounds.
Additionally, the study found that the low-concentration DMSO improved blood circulation in the wound, which also contributes to faster healing.
There is reasonable evidence arising from this study that suggests low-concentration DMSO has the potential to be a valuable tool in the treatment of skin wounds. Further research is needed to determine the optimal concentration and application method of DMSO for use in human wound healing.
DMSO FAQs
Is DMSO Harmful to Humans?
The safety of dimethyl sulfoxide in humans is still a subject of controversy and ongoing research.
Some studies suggest that topical and oral use of it at recommended doses is generally safe, while others report adverse effects such as skin irritation, nausea, headaches, and an altered sense of taste.
There is also limited research on its long-term effects of it, so it is important to consult a healthcare professional before using it.
Additionally, some sources suggest that it should not be used internally without proper medical supervision, as it has the potential to interact with other medications and cause serious health problems.
Can Humans use DMSO for Arthritis?
Dimethyl sulfoxide is sometimes used to treat the symptoms of arthritis, although many believe that its effectiveness is still widely regarded as scientifically unproven.
It is a strong anti-inflammatory agent and has been used to reduce inflammation and swelling in the joints.
However, there is some potential risk associated with using it, including skin irritation, nausea, and headaches. Therefore, it is important to consult a doctor before using this to treat arthritis.
Is DMSO Anti-Inflammatory?
Dimethyl sulfoxide has been shown to have anti-inflammatory effects in various studies, including in whole human blood and in mouse models.
Its anti-inflammatory effects are associated with the inhibition of certain signaling pathways in human monocytes and a reduction in the production of inflammatory mediators.
However, more research is needed to fully understand the potential benefits and risks of using it for treating inflammation in humans.
How Often can you Apply DMSO on Humans?
The frequency of dimethyl sulfoxide application in humans is not well established and can vary depending on the individual and the condition being treated.
It is important to consult with a healthcare professional before using it, as they can determine the appropriate frequency and duration of treatment based on individual needs and health status.
Why is DMSO used as a Solvent?
Dimethyl sulfoxide is used as a solvent due to its ability to dissolve many substances, including organic and inorganic compounds, salts, and polar compounds.
It has a high polarity, which allows it to dissolve substances that are not soluble in water or other common solvents. This property makes it useful in a variety of applications, including in the manufacture of pharmaceuticals, in scientific research, and as a solvent for paints and inks.
Additionally, it is relatively inexpensive and has low toxicity, making it a preferred choice for many applications.
What Products Contain DMSO?
Dimethyl sulfoxide is a solvent used in a variety of products, including:
• Industrial solvents for paint, resin, and rubber
• Pharmaceutical products as a penetration enhancer for other drugs
• Topical creams and gels for the treatment of pain and inflammation
• Animal feed additives, and
• Agricultural products for plant growth and seed treatment.
It is important to note that not all of these products are intended for use on humans and that proper safety precautions should be taken when using them.
Why is DMSO used in PCR tests?
Dimethyl sulfoxide is used in PCR tests as a solvent for Taq polymerase, which is the enzyme used in polymerase chain reactions (PCRs) to amplify DNA sequences.
It is commonly used because it has the ability to dissolve a wide range of substances, including the enzymes and other components used in PCR reactions.
Its high solubility of it makes it an ideal solvent for Taq polymerase, as it allows for the efficient mixing of the enzyme with the other reaction components. This contributes to the overall efficiency and reproducibility of the PCR reaction.
Will DMSO Kill Toenail Fungus?
The use of dimethyl sulfoxide as a treatment for toenail fungus is a topic of debate among healthcare professionals. Some proponents of the liquid use it as a home remedy for toenail fungus, while others believe that its effectiveness has not been proven.
However, there is limited scientific evidence to support the use of it for the treatment of toenail fungus, and more research is needed to determine its effectiveness and safety.
It’s also important to note that self-treating a condition with dimethyl sulfoxide can be risky and should be done under the supervision of a healthcare professional.
Will DMSO Kill Ringworm?
Dimethyl sulfoxide is often used as a topical solution to treat various skin conditions, including ringworm. Some studies have suggested that it can be effective in treating ringworm, but there is limited scientific evidence to support this.
It is always recommended to consult a healthcare provider before using any new treatment, especially if you have any underlying medical conditions or are taking any medications. Your healthcare provider can provide the best guidance on how to use dimethyl sulfoxide and determine if it is safe and effective for you.
How do I get rid of DMSO?
Dimethyl sulfoxide can be removed from the skin using mild soap and warm water. It can also be mixed with a moisturiser, such as an aloe vera gel, and applied to the skin to help reduce the irritant effects.
It is important to note that it is rapidly absorbed by the skin, so it is important to wash it off as soon as possible to avoid any potential side effects.
Additionally, it is important to always wear protective gloves when handling it to avoid any potential skin irritation or contact dermatitis.
What are the Alternatives to DMSO?
There are several alternatives to dimethyl sulfoxide including:
• Ethanol – commonly used as a solvent in various applications, including cosmetics and pharmaceuticals.
• Glycerol – a clear, odorless, viscous liquid that is often used as a solvent, emollient, and sweetener.
• Propylene glycol – used as a solvent and as a humectant in personal care products and pharmaceuticals.
• Polyethylene glycol (PEG) – a polymer used as a solvent and emulsifier in various industries.
• N-Methyl-2-pyrrolidone (NMP) – a polar solvent used in various industrial applications.
It’s important to note that the suitability of these alternatives depends on the specific application and further research may be required before use.
Which is Better DMSO or MSM?
When choosing between DMSO or methylsulfonylmethane (MSM), depends on the individual and their specific needs.
Dimethyl sulfoxide is a powerful antioxidant and anti-inflammatory with a wide range of health benefits, while MSM is a naturally occurring dietary supplement with similar health benefits.
Both substances can be used to treat pain, inflammation, and other health issues, but they each have their own unique properties and benefits.
Ultimately, it is up to the individual to decide which one is best for their needs.
Is DMSO Safe during Pregnancy?
It would be advisable to speak with a professional who is qualified to provide advice on this. However, based on the findings of one study which explores the effects it may have on neurochemistry and brain morphology it would be advisable to avoid any additional exposure to what is naturally occurring at this stage in life. 33
Conclusion
As this article has considered the medical and industrial applications of DMSO to gain a more complete perspective on its current use and the underpinning research I hope it helps you approach the topic from a more informed base.
I’ve personally grown up around this (I’m a 70s baby) and see it as a normal part of my Grandparent’s life. Thanks to my Wife, this has made a welcome return to our home as one of those forgotten things from long ago. 34
I have found the broad scope of use of DMSO has been both practical and beneficial in my life. I can only speak of my personal experience and reference the studies I’ve explored within my scope of capability. It is up to you to do your own research first and speak with someone who can give you one-on-one advice as a qualified expert before you take any action.
Notice
DMSO is sold on the market as a solvent for solvent-related purposes only. Any alternative uses of this product that you may choose to apply it to are done so at your own risk. We advise that suitably qualified expert guidance for any alternative applications is a must.
None of the content here is in any way an endorsement or encouragement for the unguided use or self-administration of DMSO, or any other products, in any form or manner.
This content is provided without warranty and is for informational and educational purposes only. It does not seek to replace the personal relationship and connection you have with your trusted Doctor or Healthcare Professional.
Our intention here is to support your understanding of what this is, and your ability to participate in conversations with suitably qualified experts relating to DMSO uses should you need to be involved in one.
Ok, I've read the notice. Take me back to the top, please.
References
- “Dimethyl sulfoxide” – Royal Society of Chemistry Staff, Last Checked 12 January 2023 [ChemSpider] [Archive] ↩︎
- “DMSO (Dimethyl sulfoxide)” – COC Staff, Last Checked 12 January 2023 [COC] [Archive] ↩︎
- “Amplification of anticancer efficacy by co-delivery of doxorubicin and lonidamine with extracellular vesicles” – H. Li, W. Xu, F. Li, R. Zeng, X. Zhang, X. Wang, S. Zhao, J. Weng, Z. Li, L. Sun, 16 October 2021 [Taylor & Francis] [Archive] ↩︎
- “Dimethyl Sulfoxide” – K. Capriotti, J. A. Capriotti, September 2012 [PubMed] [Archive] ↩︎
- “Enhanced Skin Permeation of Estradiol by Dimethyl Sulfoxide Containing Transdermal Patches” – A. Otterbach, A. Lamprecht, 1 March 2021 [MDPI] [Archive] ↩︎
- “In Vitro Evaluation of the Combination of Melaleuca alternifolia (Tea Tree) Oil and Dimethyl Sulfoxide (DMSO) against Trophozoites and Cysts of Acanthamoeba Strains. Oxygen Consumption Rate (OCR) Assay as a Method for Drug Screening” – T. Martín-Pérez, I. Heredero-Bermejo, C. Verdú-Expósito, J. Pérez-Serrano, 19 April 2021 [MDPI] [Archive] ↩︎
- “Dimethyl Sulfoxide” – K. Capriotti, J. A. Capriotti, September 2012 [PubMed] [Archive] ↩︎
- “Adverse reactions of dimethyl sulfoxide in humans: a systematic review” – B. Kollerup Madsen, M. Hilscher, D. Zetner, J. Rosenberg, 6 August 2019 [PubMed] [Archive] ↩︎
- “Characterization of increased drug metabolism activity in dimethyl sulfoxide (DMSO)-treated Huh7 hepatoma cells” – S. Choi, B. Sainz Jr., P. Corcoran, S. Uprichard, H. Jeong, 13 October 2010 [PubMed] [Archive] ↩︎
- “The Safety, Pharmacokinetics, and Efficacy of Intraocular Celecoxib” – S. J. Kim, H. Toma, R. Shah, U. B. Kompella, S. K. Vooturi, J. Sheng, 10 March 2014 [PubMed] [Archive] ↩︎
- “Comparative cytotoxic effects of methanol, ethanol and DMSO on human cancer cell lines” – S. T. Nguyen, H. T. L. Nguyen, K. D. Truong, 29 July 2020 [BMRAT] [Archive] ↩︎
- “Strategies in developing dimethyl sulfoxide (DMSO)-free cryopreservation protocols for biotherapeutics” – M. D. Ekpo, G. F. Boafo, J. Xie, X. Liu, C. Chen, S. Tan, 5 October 2022 [Frontiers in Immunology] [Archive] ↩︎
- “Amplification of anticancer efficacy by co-delivery of doxorubicin and lonidamine with extracellular vesicles” – H. Li, W. Xu, F. Li, R. Zeng, X. Zhang, X. Wang, S. Zhao, J. Weng, Z. Li, L. Sun, 16 October 2021 [Taylor & Francis] [Archive] ↩︎
- “Betaine, Dimethyl Sulfoxide, and 7-Deaza-dGTP, a Powerful Mixture for Amplification of GC-Rich DNA Sequences” – M. Musso, R. Bocciardi, S. Parodi, R. Ravazzolo, I. Ceccherini, 8 November 2006 [PubMed] [Archive] ↩︎
- “Dimethyl sulfoxide (DMSO) as the sulfur source for the production of desulfurizing resting cells of Gordonia alkanivorans RIPI90A” – G. Mohebali, A. S Ball, A. Kaytash, B. Rasekh, March 2008 [PubMed] [Archive] ↩︎
- “Oxidative Dissolution of Metals in Organic Solvents” – X. Li, K. Binnemans, 16 March 2021 [PubMed] [Archive] ↩︎
- “Refining of crude sulfate turpentine obtained from a kraft pulp mill: A pilot scale treatment” – A. E. B. Dal, 20 October 2021 [Semantic Scholar] [Archive] [NC State University] [Archive] ↩︎
- “AP 42, Fifth Edition, Volume I: Chapter 10: Wood Products Industry – 10.2 Chemical Wood Pulping” – Author, September 1990 [EPA – Link to Final Section] [Archive] ↩︎
- “Dimethyl Sulfoxide Assisted Ionic Liquid Pretreatment of Switchgrass for Isoprenol Production” – S. Wang, W. Zhao, T. S. Lee , S. W. Singer, B. A. Simmons, S. Singh, Q. Yuan, G. Cheng, 25 January 2018 [ACS] [Archive] ↩︎
- “Dimethyl Sulfoxide Enhances Effectiveness of Skin Antiseptics and Reduces Contamination Rates of Blood Cultures” – J. J. Tarrand, P. R. LaSala, X. Y. Han, K. V. Rolston, D. P. Kontoyiannisb, May 2012 [PubMed] [Archive] ↩︎
- “Intravesical drug delivery for dysfunctional bladder” – C. C. Hsu, Y. C. Chuang, M. B. Chancellor, 22 January 2013 [Wiley] [Archive] ↩︎
- “Prolonged activity nicotine patch” – R. W. Baker, F. Kochinke, C. Huang, 28 October 1988 [Google Patents] [Archive] ↩︎
- “Enhanced Skin Permeation of Estradiol by Dimethyl Sulfoxide Containing Transdermal Patches” – A. Otterbach, A. Lamprecht, 4 January 2021 [MDPI] [Archive] ↩︎
- “Say No to DMSO: Dimethylsulfoxide Inactivates Cisplatin, Carboplatin and Other Platinum Complexes” – M. D. Hall, K. A. Telma, K. E. Chang, T. D. Lee, J. P. Madigan,1 J. R. Lloyd, I. S. Goldlust, J. D. Hoeschele, M. M. Gottesman, 8 May 2014 [PubMed] [Archive] ↩︎
- “Variation in dimethyl sulfoxide use in stem cell transplantation: a survey of EBMT centers” – P. Windrum, T. C. M. Morris, M. B. Drake, D. Niederwieser, T. Ruutu, 25 July 2005 [Nature] [Archive] ↩︎
- “The role of depletion of dimethyl sulfoxide before autografting: on hematologic recovery, side effects, and toxicity” – R. Syme, M. Bewick, D. Stewart, K. Porter, T. Chadderton, S. Glück, 15 August 2003 [ScienceDirect] [Archive] ↩︎
- “Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity?” – M. Awan, I. Buriak, R. Fleck, B. Fuller, A. Goltsev, J. Kerby, M. Lowdell, P. Mericka, A. Petrenko, Y. Petrenko, O. Rogulska, A. Stolzing, G. N. Stacey, 28 April 2020 [Future Medicine] [Archive] ↩︎
- “DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro” – M. Verheijen, M. Lienhard, Y. Schrooders, O. Clayton, R. Nudischer, S. Boerno, B. Timmermann, N. Selevsek, R. Schlapbach, H. Gmuender, S. Gotta, J. Geraedts, R. Herwig, J. Kleinjans, F. Caiment, 15 March 2019 [Scientific Reports] [Archive] ↩︎
- “2.2 Dimethyl sulfoxide (DMSO)” – TGA Staff, 10 September 2018 [TGA] [Archive] ↩︎
- “Dimethyl Sulfoxide-Free Cryopreservation of Human Umbilical Cord Mesenchymal Stem Cells Based on Zwitterionic Betaine and Electroporation” – L. Gao, Q. Zhou, Y. Zhang, S. Sun, L. Lv, P. Ma, J. Yang, M. Liu, L. Zhang, X. Wang, L. Zhan, 5 June 2021 [MDPI] [Archive] ↩︎
- “DMSO Represses Inflammatory Cytokine Production from Human Blood Cells and Reduces Autoimmune Arthritis” – I. Elisia, H. Nakamura, V. Lam, E. Hofs, R. Cederberg, J. Cait, M. R. Hughes, L. Lee W. Jia, H. H. Adomat, E. S. Guns, K. M. McNagny, I. Samudio, G. Krystal, 31 March 2016 [PubMed] [Archive] ↩︎
- “Low‐concentration DMSO accelerates skin wound healing by Akt/mTOR‐mediated cell proliferation and migration in diabetic mice” – W. Guo, W. Qiu, X. Ao, W. Li, X. He, L. Ao, X. Hu, Z. Li, M. Zhu, D. Luo, W. Xing, X. Xu, 7 April 2020 [PubMed] [Archive] ↩︎
- “Exposure to DMSO during infancy alters neurochemistry, social interactions, and brain morphology in long-evans rats” – Z. Rabow, T. Morningstar, M. Showalter, H. Heil, K. Thongphanh, S. Fan, J. Chan, V. M. Cerdeño, R. Berman, D. Zagzag, E. Nudler, O. Fiehn, M. Lechpammer, 10 April 2021 [Wiley] [Archive] ↩︎
- “Molecule of the Week Archive: Dimethyl sulfoxide” – ACS Staff, 20 September 2021 [ACS] [Archive] ↩︎
Last Updated on 5 months by D&C Editorial Team


